







healing power
Announcement: Shipping Delays from June 20th to 25th, 2024
Dear Valued Customers, We'd like to inform you that there might be delays in shipping from June 20th to 25th, 2024. If you wish to receive your order sooner, we kindly ask you to place it before June 19th. Rest assured, orders placed by this date will be shipped out on the same business day within our working hours.
Made in Japan with a worldwide patent






And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.



[ TRIPLE ]




This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth. M-Lollies is a hard candy with mild milky taste. While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who cannot swallow capsules.



M-Powder is recommended for those who cannot swallow capsules, and/or who would like to have it without water on the go.
the mouth and is absorbed quickly and efficiently.
individual stick type.
Consume without water,
put straight to the mouth and slowly
let it melt.

![MAF CAPSULES [TRIPLE]](http://saisei-pharma.co.jp/en/wp-content/uploads/sites/3/3c6fa9fd97eafa80feaf066e64eceed2.png)



MAF CAPSULES TRIPLE
¥20.000

Latest product update
Starting from August 1st, 2023 we are thrilled to announce that we have new stock of M-powder [Best Before 30/Jun/2025]. The regular price is 14,000 yen. Order the old stock of M-Powder now and receive 50% OFF until 31/Oct/2023, while stocks last.
Boosting your immunity with MAF Series
MAF = Macrophage Activating Factor
Our MAF Series boosts your immunity by activating the immune cells without any side effects and help you stay young and healthy. The MAF Series is made exclusively with natural ingredients and produced in our GMP-certified factory (Certification #26518) in Osaka, Japan.

Anti-aging and longevity
Saisei Mirai’s products target to enhance appearance and health; the goal is to look 20 years younger and to live healthy for over 100 years. We are programmed to get old but there are ways to slow down the process.
Macrophages play an important role in addressing anti-aging by supporting the immune system and eliminating diseased and damaged cells. It helps with tissue repair, rejuvenation, regeneration, defending infections and tumor growth.
The roles of macrophage
What are macrophages?
– Stimulate cell activities of the immune system.
– Promote wound healing and skin repair.
– Promote hair regeneration.
– Phagocytose bacteria and destroy virus-infected cells.
– Ingest and destroy microorganisms.
– Degrade and remove dead cells.


Health
Protect against infections

Lonvevity
Live longer and healthier

Youth
Slow down aging

Energy
Fight fatigue

Beauty
Improve skin conditions

GcMAF - Discover the effects
Provided by numerous clinical trials!
Approved as food/supplement in various countries and patented worldwide.
GcMAF has proved its effects in the medical fields around the world. GcMAF is acknowledged for its function to help the recovery from serious illness such as cancer and autism, and recently found in clinical trials in Ukraine, to have positive effects on patients with Covid-19.

- Macrophage Activating Factor Vitamin D-
MAF Series
*Macrophage Activating Factor Vitamin D – MAF series are Saisei Mirai Clinic’s very own products which we researched, developed and produce.

Free of
Wheat, soy, egg, rice, gluten, starch, sugar*, hormones, antibiotics, preservatives, artificial sweeteners, artificial colors.
*Sugar is contained in M-Lollies

Topical and Oral
MAF is available in both topical and oral supplement forms. The 4 types of MAF products allows MAF to be absorbed from the mouth, intestines and the skin. The MAF Series is made exclusively with natural ingredients.

Uniqueness
MAF was developed using our own unique patented method. MAF helps boost the immune system by activating macrophages without any side effects.

Factory in Japan
MAF Series is produced in our Saisei Mirai GMP factory in Moriguchi, Osaka. The factory is certified by the Ministry of Health, Labor and Welfare of Japan.
Frequently Asked Questions
MAF Capsules Triple is our new and improved version of MAF version of MAF Capsules; it contains 3 times the active ingredient!!
Product Comparison:
- One capsule of MAF Capsules Triple contains MAF equivalent to 300ng.
- One capsule of MAF Capsules contains MAF equivalent to 100ng
- One candy of M-Lollies contains MAF equivalent to 100ng.
- One sachet of M-Powder contains MAF equivalent to 100ng.
Macrophages (Greek: big eaters) are cells originating from monocytes , a type of white blood cell found in our body. Macrophages function in both non-specific defense (innate immunity) as well as help initiate specific defense mechanisms (adaptive immunity) of vertebrate animals.
Their role is to phagocytose (engulf and then digest) cellular debris and pathogens, either as stationary or as mobile cells. They also stimulate lymphocytes and other immune cells to respond to pathogens. They are specialized phagocytic cells that attack foreign substances, infectious microbes and cancer cells through destruction and ingestion.
- Stimulate the cell activities of the immune system.
- Promote wound healing and skin repair.
- Promote hair regeneration.
- Phagocytose bacteria and destroy virus-infected cells.
- Ingest and destroy microorganisms.
- Degrade and remove dead cells.
Saisei Mirai is a medical organisation in Osaka, Japan with the purpose of treating patients and developing and producing therapies, in particular immunotherapies such as GcMAF.
We work with clinics and doctors round the world, as well as with various universities conducting clinical trials, research and developments.
Made in Japan with a worldwide patent

About Saisei Pharma
Saisei Pharma is a bio venture company established at Tokushima university in 2014.
We develop, research and manufacture immune activating food products “MAF Series (Capsule, Spray, Candy, Powder)”, ultrasonic sensitizers and electric field therapy (TTF, ECCT) etc.
Our online store offers our Immune activating food products “MAF series” to domestic and overseas customers.
We are now located in Osaka, and our products are produced in a GMP certified factory in Moriguchi, Osaka.
As a Saisei Mira Group, we cooperate with each clinic and will continue our research and development aiming to treat many diseases such as acute infection, chronic infection, cancer, autism, chronic fatigue syndrome, multiple sclerosis, rheumatoid arthritis, Alzheimer’s disease, dementia and so on.
Saisei Pharma MAF Series
Saisei Mirai Group
We have partnered with 5 domestic clinics in Japan which are based on Saisei Mirai Medical Corporation, bio-venture companies, pharmacies, and universities and medical institutions in Japan and overseas, and have started providing advanced medical care worldwide.
Saisei Pharma products
Macrophage activating factor Vitamin D
MAF series
The MAF Series will help boost the immune system by activating immune cells without any side effects.
This anti-aging support food is for those who would like to stay young and healthy. The MAF Series is made with natural ingredients and produced in our GMP Certified factory (Certification No. 26518) in Osaka.

Ultrasonic Sensitizers
We have developed our very own Ultrasonic Sensitizers for Sonodynamic Therapy (SDT) which are used at Saisei Mirai Clinics. The sensitizer is taken up only by cancer cells, and the ultrasound energy beam activates the sensitizer to destroy only the cancer cells. Based on our own research, we have developed the latest non-toxic sensitizers.

Electric Field Therapy
Saisei Mirai Clinics offers Electric Field Therapy (TTF, ECCT). We have Blanket, Vest and Cap type applicators. The size and electric field is customized to suit each patient’s disease and lesion. Treatment is conducted at home and the electric field is adjusted and results are checked with a CT and/or MRI scan once every 1 to 3 months.

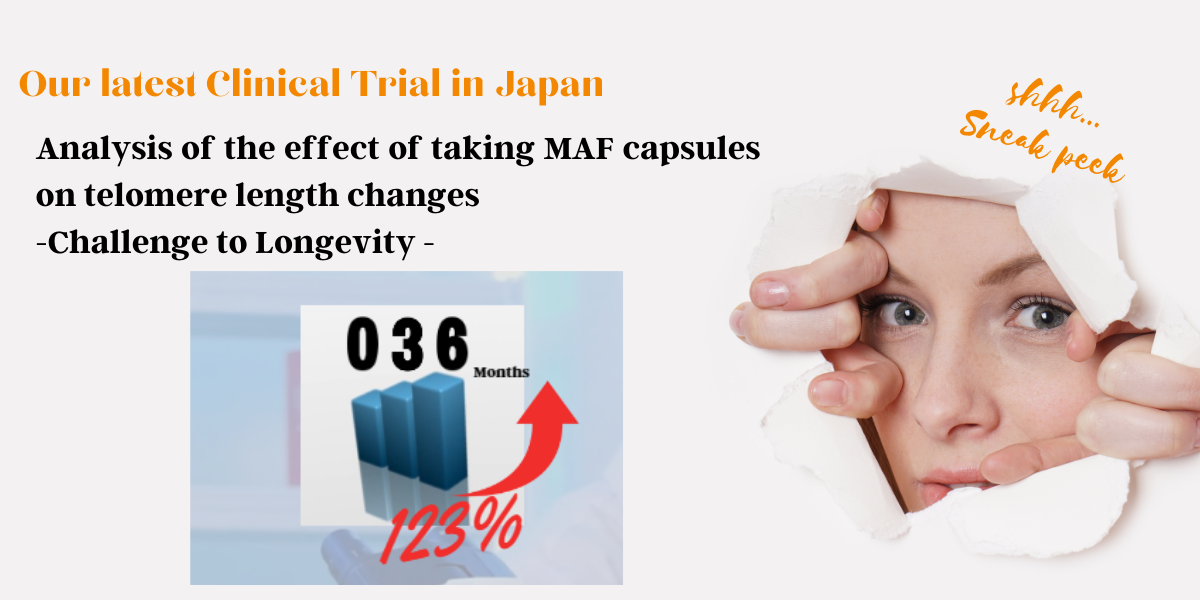
Clinical trials using MAF for hospitalized COVID-19 patients

Lancet SSRN Preprints
The Effect of MAF Capsules and M Capsules on Lymphopenia and Clinical Outcomes in Non-Critical Hospitalized COVID-19 Patients.
US National Library of Medicine,
NIH clinical trial
Trial Efficacy of Saisei Pharma Dietary Supplements MAF Capsules, 148 mg and M Capsules, 148 mg in Hospitalized COVID-19 Patients (SaiseiCovUKR)

Kazakhstan
The clinical trial using MAF capsules has started in Kazakhstan for the patients suffering with coronavirus aftereffects in February 2021.
Ukraine
Update on the clinical trial using MAF for hospitalized COVID-19 patients in Ukraine

Italy
The clinical trial using MAF capsules has started in Italy from November for hospitalised patients with COVID-19.

Ukraine
The clinical trial using MAF capsules has started in Ukraine from the end of October for hospitalised patients with COVID-19.
Rationales of MAF as a therapeutic target for SARS-CoV-2
The fact that around 96% of patients recover from the infection

Potential role of GcMAF
Potential role of GcMAF in suppressing the severity of COVID-19-induced immune responses: Lesson learned from HIV
Autism spectrum disorder and GcMAF immunotherapy
Product Campaign!
Untill 31/Oct/2023


7,000 JPY
4,250 JPY

14,000JPY
8,500 JPY
M POWDER 50% OFF
[Best Before Date: 30/Nov/2023]
M LOLLIES 50% OFF
[Best Before Date: 31/Dec/2023]

The product was used in Ukraine Clinical Trial and has proven excellent results for hospitalized covid-19 patients.
CLINICAL STUDY MAF capsules efficacy in post-COVID syndrome treatment is started in KAZAKHSTAN
CLINICAL STUDY MAF capsules efficacy in post-COVID syndrome treatment is started in KAZAKHSTAN
MedInc Ltd in Kazakhstan on the base of National Research Cardiac Surgery Center has started the implementation of prospective randomized open-label clinical trial: “Evaluation of immune modulator MAF capsules efficacy in a therapy of patients with post-COVID syndrome”. The study sponsored by the developer and manufacturer of the study product Saisei Pharma Co. Ltd, Japan.
The goal of the study is the evaluation of the safety and efficiency of a MAF capsules dietary supplement with anti-inflammatory immune response modulating function in combined treatment of the post-COVID syndrome. |
The trial started in February 2021 and targeted 200 patients with post-COVID syndrome, which will be randomized 1:1 in two groups:
Group 1: SOC (standard of care) + dietary supplement MAF capsules administration for 30 days.
Group 2: SOC
The study primary endpoints aim to evaluate the improvement within 30 days period after MAF capsules administration, where the clinical efficacy refers to an improvement of life quality according to Chalder Fatigue Scale and the “Postcovid syndrome” consequences and symptoms severity evaluation according to ISARIC questionnaire.
The study secondary endpoints included the evaluation of changes according to the Karnofsky Performance Scale and Numerical Rating Scale, and evaluation of the MAF capsules side effects.
The exploratory endpoints include effects on:
1. Lymphocyte count, T cells and its subpopulation count, and B cells count
2. The plasma level of IgG against SARS-CoV-2
Clinical trial using MAF for hospitalized COVID-19 patients in Ukraine
In June 2020 we applied to the COVID-19 Scientific Technical Triage of the US FDA for the evaluation of the rationale to study the efficacy of MAF Capsules in COVID-19 treatment. The US FDA in PreIND 151946 meeting response recommended a small proof of concept (POC) study as the initial step prior to the large-scale trial be run. The US FDA indicated recommendations including the major study endpoints addressing the investigation of MAF Capsules efficacy as a potential new drug was implemented in the proposed study design. The recommended efficacy endpoints were also implemented in the open-label randomized clinical trial that started in Ukraine in November 2020 to assess the efficacy and safety of dietary supplements MAF Capsules, 148 mg and M Capsules, 148 mg in addition to standard of care (SOC) compared with SOC in the treatment of hospitalized non-critical COVID-19 patients.Summary of preliminary results of clinical study
The ongoing interim study results showed a decrease in all-cause mortality and necessity of oxygen supply, as the mortality was 4/15 in the control group, vs 0/16 and 1/17 of currently enrolled patients in the MAF Capsules and M Capsules groups respectively. The mean duration of supplemental oxygen was 8.5 days in the control group, vs 4.1 and 5.1 days in the MAF Capsules and M Capsules groups respectively. MAF Capsules, which is under investigational new drug process, is a dietary supplement that targets guts mucosal immunity to modulate macrophages functionality, limiting epithelial damage, and controlling inflammation response during COVID-19.Clinical trial groups:
- Control group
- MAF Capsules (colostrum MAF) group
- M Capsules (whey MAF) group
Patients randomized 1:1:1 to:
- SOC (standard of care)
- SOC plus MAF Capsules (148 mg, 3 caps. TID for 14 days)
- SOC plus M Capsules (148 mg, 3 caps. TID for 14 days)
As compared to the control, both MAF groups showed a clear trend in decreasing mortality
- No adverse events
- Decrease in the mortality rate
- Decrease in necessity and duration of supplemental oxygen
- Decrease in time to recovery
- Decrease in time until hospital discharge
- Preventing of respiratory failure
- Restoring the base-line decreased lymphocytes count

US National Library of Medicine, NIH clinical trial
Trial Efficacy of Saisei Pharma Dietary Supplements MAF Capsules, 148 mg and M Capsules, 148 mg in Hospitalized COVID-19 Patients (SaiseiCovUKR)
URL : https://clinicaltrials.gov/ct2/show/NCT04762628
Kazakhstan
The clinical trial using MAF capsules has started in Kazakhstan for the patients suffering with coronavirus aftereffects in February 2021.

Ukraine
Update on the clinical trial using MAF for hospitalized COVID-19 patients in Ukraine.
Italy
The clinical trial using MAF capsules has started in Italy from November for hospitalised patients with COVID-19.
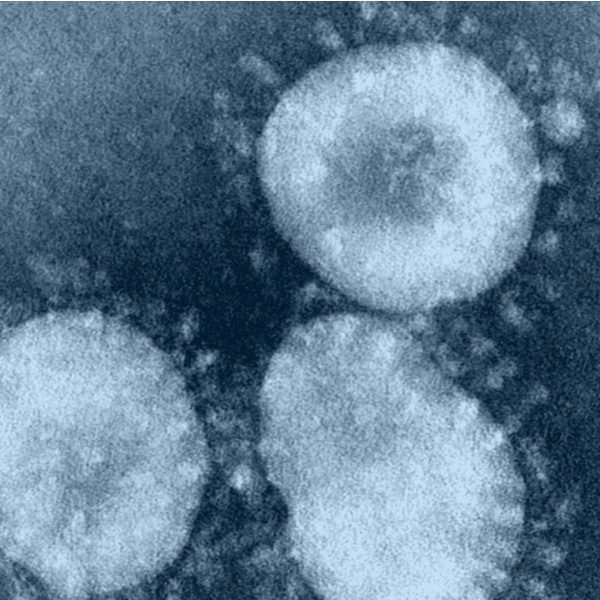
Ukraine
The clinical trial using MAF capsules has started in Ukraine from the end of October for hospitalised patients with COVID-19.

Rationales of MAF as a therapeutic target for SARS-CoV-2
The fact that around 96% of patients recover from the infection

Potential role of GcMAF
Potential role of GcMAF in suppressing the severity of COVID-19-induced immune responses: Lesson learned from HIV

Saisei Pharma plans clinical study of oral MAF in COVID-19 patients
[Read more..]
Please check the below URL.
[finanzen.ch] [advfn.com] [thechestnutpost.com] [benzinga.com]
3rd Generation GcMAF was developed by Saisei Pharma for which we hold patents. There are fake GcMAF products sold online in Australia by a company which is registered in Hong Kong. Please be aware that 3rd Generation GcMAF products are only produced by Saisei Pharma. Any other products from other companies are fake products.

media listing information
The decision is based on published preclinical evidence, preliminary reports from independent studies and is supported by data of the safety and efficacy of oral MAF in conditions that require the involvement of macrophages as the front line defense in innate immunity. [Read more..]
Our Press Release featured on the following sites:
[finanzen.ch] [advfn.com] [benzinga.com]

Saisei Pharma
plans clinical study of oral MAF in COVID-19 patients
M Lollies & M Powder [50% OFF] while stocks last
M Lollies & M Powder [50%OFF Short Dated Sale] while stocks lastMAF Capsules and M-Lollies are approved as food supplements





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.



[ TRIPLE ]





This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth. M-Lollies is a hard candy with mild milky taste. While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who cannot swallow capsules.




M-Powder is recommended for those who cannot swallow capsules, and/or who would like to have it without water on the go.
the mouth and is absorbed quickly and efficiently.
individual stick type.
Consume without water,
put straight to the mouth and slowly
let it melt.

![MAF CAPSULES [TRIPLE]](http://saisei-pharma.co.jp/en/wp-content/uploads/sites/3/3c6fa9fd97eafa80feaf066e64eceed2.png)




- Macrophage Activating Factor Vitamin D-
MAF Series
*Macrophage Activating Factor Vitamin D – MAF series are Saisei Mirai Clinic’s very own products which we researched, developed and produce.

About Saisei Pharma
Saisei Pharma is a bio venture company established at Tokushima university in 2014.
We develop, research and manufacture immune activating food products “MAF Series (Capsule, Spray, Candy, Powder)”, ultrasonic sensitizers and electric field therapy (TTF, ECCT) etc.
Our online store offers our Immune activating food products “MAF series” to domestic and overseas customers.
We are now located in Osaka, and our products are produced in a GMP certified factory in Moriguchi, Osaka.
As a Saisei Mira Group, we cooperate with each clinic and will continue our research and development aiming to treat many diseases such as acute infection, chronic infection, cancer, autism, chronic fatigue syndrome, multiple sclerosis, rheumatoid arthritis, Alzheimer’s disease, dementia and so on.

Free from
Wheat, soy, egg, rice, gluten, starch, sugar (excluding M-Lollies), hormones, antibiotics, preservatives, artificial sweeteners, artificial colours.

Easy to take
1 Colostrum MAF capsule contains 1 mg of Saisei Mirai’s unique MAF (Equivalent to 100 ng of Macrophage Activating Factor) Colostrum-MAF™.

Produced in GMP Certified factory in Japan
MAF Series products are produced in our Saisei Mirai GMP Certified factory (Certification No. 26518) in Moriguchi, Osaka, certified by the Ministry of Health, Labor and Welfare of Japan.

Very unique MAF
We have developed our very own unique MAF (macrophage activating factor) using our patented method. MAF helps boost the immune system by activating macrophages without any side effects.
Saisei Pharma MAF Series
Saisei Mirai Group
We have partnered with 5 domestic clinics in Japan which are based on Saisei Mirai Medical Corporation, bio-venture companies, pharmacies, and universities and medical institutions in Japan and overseas, and have started providing advanced medical care worldwide.
Saisei Pharma products
Macrophage activating factor Vitamin D
MAF series
The MAF Series will help boost the immune system by activating immune cells without any side effects.
This anti-aging support food is for those who would like to stay young and healthy. The MAF Series is made with natural ingredients and produced in our GMP Certified factory (Certification No. 26518) in Osaka.

Ultrasonic Sensitizers
We have developed our very own Ultrasonic Sensitizers for Sonodynamic Therapy (SDT) which are used at Saisei Mirai Clinics. The sensitizer is taken up only by cancer cells, and the ultrasound energy beam activates the sensitizer to destroy only the cancer cells. Based on our own research, we have developed the latest non-toxic sensitizers.

Electric Field Therapy
Saisei Mirai Clinics offers Electric Field Therapy (TTF, ECCT). We have Blanket, Vest and Cap type applicators. The size and electric field is customized to suit each patient’s disease and lesion. Treatment is conducted at home and the electric field is adjusted and results are checked with a CT and/or MRI scan once every 1 to 3 months.

Contact us
Contact us

Made in Japan with a worldwide patent






And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.



[ TRIPLE ]





This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth. M-Lollies is a hard candy with mild milky taste. While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who cannot swallow capsules.




M-Powder is recommended for those who cannot swallow capsules, and/or who would like to have it without water on the go.
the mouth and is absorbed quickly and efficiently.
individual stick type.
Consume without water,
put straight to the mouth and slowly
let it melt.

![MAF CAPSULES [TRIPLE]](http://saisei-pharma.co.jp/en/wp-content/uploads/sites/3/3c6fa9fd97eafa80feaf066e64eceed2.png)



MAF CAPSULES TRIPLE
¥20.000

Latest product update
Starting from August 1st, 2023 we are thrilled to announce that we have new stock of M-powder [Best Before 30/Jun/2025]. The regular price is 14,000 yen. Order the old stock of M-Powder now and receive 50% OFF until 31/Oct/2023, while stocks last.
Boosting your immunity with MAF Series
MAF = Macrophage Activating Factor
Our MAF Series boosts your immunity by activating the immune cells without any side effects and help you stay young and healthy. The MAF Series is made exclusively with natural ingredients and produced in our GMP-certified factory (Certification #26518) in Osaka, Japan.
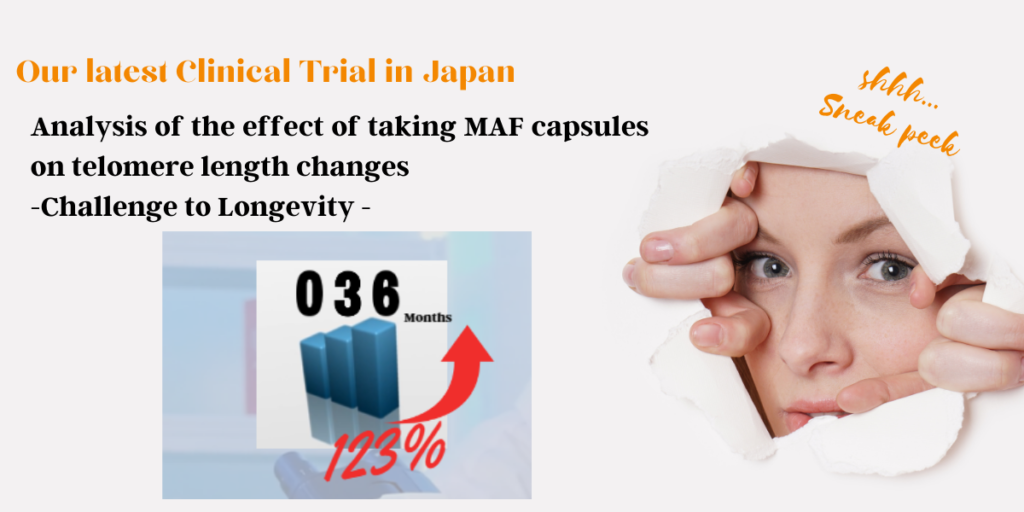

Anti-aging and longevity
Saisei Mirai’s products target to enhance appearance and health; the goal is to look 20 years younger and to live healthy for over 100 years. We are programmed to get old but there are ways to slow down the process.
Macrophages play an important role in addressing anti-aging by supporting the immune system and eliminating diseased and damaged cells. It helps with tissue repair, rejuvenation, regeneration, defending infections and tumor growth.
The roles of macrophage
What are macrophages?
– Stimulate cell activities of the immune system.
– Promote wound healing and skin repair.
– Promote hair regeneration.
– Phagocytose bacteria and destroy virus-infected cells.
– Ingest and destroy microorganisms.
– Degrade and remove dead cells.


Health
Protect against infections

Lonvevity
Live longer and healthier
Youth
Slow down aging
Energy
Fight fatigue

Beauty
Improve skin conditions

GcMAF - Discover the effects
Provided by numerous clinical trials!
Approved as food/supplement in various countries and patented worldwide.
GcMAF has proved its effects in the medical fields around the world. GcMAF is acknowledged for its function to help the recovery from serious illness such as cancer and autism, and recently found in clinical trials in Ukraine, to have positive effects on patients with Covid-19.

- Macrophage Activating Factor Vitamin D-
MAF Series
*Macrophage Activating Factor Vitamin D – MAF series are Saisei Mirai Clinic’s very own products which we researched, developed and produce.

Free of
Wheat, soy, egg, rice, gluten, starch, sugar*, hormones, antibiotics, preservatives, artificial sweeteners, artificial colors.
*Sugar is contained in M-Lollies

Topical and Oral
MAF is available in both topical and oral supplement forms. The 4 types of MAF products allows MAF to be absorbed from the mouth, intestines and the skin. The MAF Series is made exclusively with natural ingredients.

Uniqueness
MAF was developed using our own unique patented method. MAF helps boost the immune system by activating macrophages without any side effects.

Factory in Japan
MAF Series is produced in our Saisei Mirai GMP factory in Moriguchi, Osaka. The factory is certified by the Ministry of Health, Labor and Welfare of Japan.
Made in Japan with a worldwide patent








About Saisei Pharma
Saisei Pharma is a bio venture company established at Tokushima university in 2014.
We develop, research and manufacture immune activating food products “MAF Series (Capsule, Spray, Candy, Powder)”, ultrasonic sensitizers and electric field therapy (TTF, ECCT) etc.
Our online store offers our Immune activating food products “MAF series” to domestic and overseas customers.
We are now located in Osaka, and our products are produced in a GMP certified factory in Moriguchi, Osaka.
As a Saisei Mira Group, we cooperate with each clinic and will continue our research and development aiming to treat many diseases such as acute infection, chronic infection, cancer, autism, chronic fatigue syndrome, multiple sclerosis, rheumatoid arthritis, Alzheimer’s disease, dementia and so on.
Saisei Mirai Group
We have partnered with 5 domestic clinics in Japan which are based on Saisei Mirai Medical Corporation, bio-venture companies, pharmacies, and universities and medical institutions in Japan and overseas, and have started providing advanced medical care worldwide.
Saisei Pharma products
Macrophage activating factor Vitamin D
MAF series
The MAF Series will help boost the immune system by activating immune cells without any side effects.
This anti-aging support food is for those who would like to stay young and healthy. The MAF Series is made with natural ingredients and produced in our GMP Certified factory (Certification No. 26518) in Osaka.

Ultrasonic Sensitizers
We have developed our very own Ultrasonic Sensitizers for Sonodynamic Therapy (SDT) which are used at Saisei Mirai Clinics. The sensitizer is taken up only by cancer cells, and the ultrasound energy beam activates the sensitizer to destroy only the cancer cells. Based on our own research, we have developed the latest non-toxic sensitizers.

Electric Field Therapy
Saisei Mirai Clinics offers Electric Field Therapy (TTF, ECCT). We have Blanket, Vest and Cap type applicators. The size and electric field is customized to suit each patient’s disease and lesion. Treatment is conducted at home and the electric field is adjusted and results are checked with a CT and/or MRI scan once every 1 to 3 months.

Clinical trials using MAF for hospitalized COVID-19 patients

Lancet SSRN Preprints
The Effect of MAF Capsules and M Capsules on Lymphopenia and Clinical Outcomes in Non-Critical Hospitalized COVID-19 Patients.

US National Library of Medicine,
NIH clinical trial
Trial Efficacy of Saisei Pharma Dietary Supplements MAF Capsules, 148 mg and M Capsules, 148 mg in Hospitalized COVID-19 Patients (SaiseiCovUKR)

Kazakhstan
The clinical trial using MAF capsules has started in Kazakhstan for the patients suffering with coronavirus aftereffects in February 2021.

Ukraine
Update on the clinical trial using MAF for hospitalized COVID-19 patients in Ukraine

Italy
The clinical trial using MAF capsules has started in Italy from November for hospitalised patients with COVID-19.

Ukraine
The clinical trial using MAF capsules has started in Ukraine from the end of October for hospitalised patients with COVID-19.

Rationales of MAF as a therapeutic target for SARS-CoV-2
The fact that around 96% of patients recover from the infection

Potential role of GcMAF
Potential role of GcMAF in suppressing the severity of COVID-19-induced immune responses: Lesson learned from HIV
Product Campaign!
Untill 31/Oct/2023


7,000 JPY
4,250 JPY

14,000JPY
8,500 JPY
M POWDER 50% OFF
[Best Before Date: 30/Nov/2023]
M LOLLIES 50% OFF
[Best Before Date: 31/Dec/2023]

The product was used in Ukraine Clinical Trial and has proven excellent results for hospitalized covid-19 patients.
CLINICAL STUDY MAF capsules efficacy in post-COVID syndrome treatment is started in KAZAKHSTAN
CLINICAL STUDY MAF capsules efficacy in post-COVID syndrome treatment is started in KAZAKHSTAN
MedInc Ltd in Kazakhstan on the base of National Research Cardiac Surgery Center has started the implementation of prospective randomized open-label clinical trial: “Evaluation of immune modulator MAF capsules efficacy in a therapy of patients with post-COVID syndrome”. The study sponsored by the developer and manufacturer of the study product Saisei Pharma Co. Ltd, Japan.
The goal of the study is the evaluation of the safety and efficiency of a MAF capsules dietary supplement with anti-inflammatory immune response modulating function in combined treatment of the post-COVID syndrome. |
The trial started in February 2021 and targeted 200 patients with post-COVID syndrome, which will be randomized 1:1 in two groups:
Group 1: SOC (standard of care) + dietary supplement MAF capsules administration for 30 days.
Group 2: SOC
The study primary endpoints aim to evaluate the improvement within 30 days period after MAF capsules administration, where the clinical efficacy refers to an improvement of life quality according to Chalder Fatigue Scale and the “Postcovid syndrome” consequences and symptoms severity evaluation according to ISARIC questionnaire.
The study secondary endpoints included the evaluation of changes according to the Karnofsky Performance Scale and Numerical Rating Scale, and evaluation of the MAF capsules side effects.
The exploratory endpoints include effects on:
1. Lymphocyte count, T cells and its subpopulation count, and B cells count
2. The plasma level of IgG against SARS-CoV-2
Clinical trial using MAF for hospitalized COVID-19 patients in Ukraine
In June 2020 we applied to the COVID-19 Scientific Technical Triage of the US FDA for the evaluation of the rationale to study the efficacy of MAF Capsules in COVID-19 treatment. The US FDA in PreIND 151946 meeting response recommended a small proof of concept (POC) study as the initial step prior to the large-scale trial be run. The US FDA indicated recommendations including the major study endpoints addressing the investigation of MAF Capsules efficacy as a potential new drug was implemented in the proposed study design. The recommended efficacy endpoints were also implemented in the open-label randomized clinical trial that started in Ukraine in November 2020 to assess the efficacy and safety of dietary supplements MAF Capsules, 148 mg and M Capsules, 148 mg in addition to standard of care (SOC) compared with SOC in the treatment of hospitalized non-critical COVID-19 patients.Summary of preliminary results of clinical study
The ongoing interim study results showed a decrease in all-cause mortality and necessity of oxygen supply, as the mortality was 4/15 in the control group, vs 0/16 and 1/17 of currently enrolled patients in the MAF Capsules and M Capsules groups respectively. The mean duration of supplemental oxygen was 8.5 days in the control group, vs 4.1 and 5.1 days in the MAF Capsules and M Capsules groups respectively. MAF Capsules, which is under investigational new drug process, is a dietary supplement that targets guts mucosal immunity to modulate macrophages functionality, limiting epithelial damage, and controlling inflammation response during COVID-19.Clinical trial groups:
- Control group
- MAF Capsules (colostrum MAF) group
- M Capsules (whey MAF) group
Patients randomized 1:1:1 to:
- SOC (standard of care)
- SOC plus MAF Capsules (148 mg, 3 caps. TID for 14 days)
- SOC plus M Capsules (148 mg, 3 caps. TID for 14 days)
As compared to the control, both MAF groups showed a clear trend in decreasing mortality
- No adverse events
- Decrease in the mortality rate
- Decrease in necessity and duration of supplemental oxygen
- Decrease in time to recovery
- Decrease in time until hospital discharge
- Preventing of respiratory failure
- Restoring the base-line decreased lymphocytes count
3rd Generation GcMAF was developed by Saisei Pharma for which we hold patents. There are fake GcMAF products sold online in Australia by a company which is registered in Hong Kong. Please be aware that 3rd Generation GcMAF products are only produced by Saisei Pharma. Any other products from other companies are fake products.

media listing information
The decision is based on published preclinical evidence, preliminary reports from independent studies and is supported by data of the safety and efficacy of oral MAF in conditions that require the involvement of macrophages as the front line defense in innate immunity. [Read more..]
Our Press Release featured on the following sites:
[finanzen.ch] [advfn.com] [benzinga.com]

Saisei Pharma
plans clinical study of oral MAF in COVID-19 patients
M Lollies & M Powder [50% OFF] while stocks last
M Lollies & M Powder [50%OFF Short Dated Sale] while stocks lastMAF Capsules and M-Lollies are approved as food supplements





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.





And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.
And it is known that plenty of macrophages exist in the Peyer's patches. MAF CAPSULES aims to activate these macrophages directly.



[ TRIPLE ]





This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth.
M-Lollies is a hard candy with mild milky taste.
While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who
cannot swallow capsules.
This is called Waldeyer's ring which protects body by bacteria and viruses coming from nose and mouth. M-Lollies is a hard candy with mild milky taste. While enjoying this delicious lolly, the immune activating factor will be absorbed by lymphoid tissue. This is also beneficial for those who cannot swallow capsules.




M-Powder is recommended for those who cannot swallow capsules, and/or who would like to have it without water on the go.
the mouth and is absorbed quickly and efficiently.
individual stick type.
Consume without water,
put straight to the mouth and slowly
let it melt.

![MAF CAPSULES [TRIPLE]](http://saisei-pharma.co.jp/en/wp-content/uploads/sites/3/3c6fa9fd97eafa80feaf066e64eceed2.png)




- Macrophage Activating Factor Vitamin D-
MAF Series
*Macrophage Activating Factor Vitamin D – MAF series are Saisei Mirai Clinic’s very own products which we researched, developed and produce.

About Saisei Pharma
Saisei Pharma is a bio venture company established at Tokushima university in 2014.
We develop, research and manufacture immune activating food products “MAF Series (Capsule, Spray, Candy, Powder)”, ultrasonic sensitizers and electric field therapy (TTF, ECCT) etc.
Our online store offers our Immune activating food products “MAF series” to domestic and overseas customers.
We are now located in Osaka, and our products are produced in a GMP certified factory in Moriguchi, Osaka.
As a Saisei Mira Group, we cooperate with each clinic and will continue our research and development aiming to treat many diseases such as acute infection, chronic infection, cancer, autism, chronic fatigue syndrome, multiple sclerosis, rheumatoid arthritis, Alzheimer’s disease, dementia and so on.
Saisei Pharma MAF Series
Saisei Mirai Group
We have partnered with 5 domestic clinics in Japan which are based on Saisei Mirai Medical Corporation, bio-venture companies, pharmacies, and universities and medical institutions in Japan and overseas, and have started providing advanced medical care worldwide.
Saisei Pharma products
Macrophage activating factor Vitamin D
MAF series
The MAF Series will help boost the immune system by activating immune cells without any side effects.
This anti-aging support food is for those who would like to stay young and healthy. The MAF Series is made with natural ingredients and produced in our GMP Certified factory (Certification No. 26518) in Osaka.

Ultrasonic Sensitizers
We have developed our very own Ultrasonic Sensitizers for Sonodynamic Therapy (SDT) which are used at Saisei Mirai Clinics. The sensitizer is taken up only by cancer cells, and the ultrasound energy beam activates the sensitizer to destroy only the cancer cells. Based on our own research, we have developed the latest non-toxic sensitizers.

Electric Field Therapy
Saisei Mirai Clinics offers Electric Field Therapy (TTF, ECCT). We have Blanket, Vest and Cap type applicators. The size and electric field is customized to suit each patient’s disease and lesion. Treatment is conducted at home and the electric field is adjusted and results are checked with a CT and/or MRI scan once every 1 to 3 months.























